COVID-19 pandemic posed a global threat to public health. Monoclonal antibodies (mAbs) have emerged as a promising therapeutic option against SARS-CoV-2 infection. However, the continuous evolution of SARS-CoV-2 has led to the rapid emergence of numerous variants that can evade host’s immune responses. Consequently, there is an urgent need to explore a class of mAbs that can recognize a conserved epitope.
The Spike (S) protein is mainly composed of the S1 and S2 subdomains, in which S1 contains the receptor binding domain (RBD) responsible for recognizing ACE2 to enter host cells. Many EUA (Emergency Use Authorization) mAbs neutralize the virus by blocking the binding of the RBD to ACE2. However, all these mAbs have been suspended due to the emergence of viral escape substitutions. Therefore, the identification of mAbs that can recognize conserved epitopes hold great potential for therapeutic application.
In this study published in PNAS, Gao Feng’s team from the Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, identified two human mAbs, BIOLS56 and IMCAS74 that recognize a de novo “breathing” cryptic epitope. These two mAbs, classified as RBD-8 mAbs based on epitope categories, have demonstrated broad neutralization capabilities against SARS-CoV, pangolin-origin coronaviruses, and all known variants of SARS-CoV-2. The structural analysis revealed that the RBD-8 cryptic epitope can be exposed through the S protein’s “breathing”, and the RBD-8 mAbs bind to the open form of RBD, resulting in the shedding of S1 from S protein.
Furthermore, the scientists have successfully optimized BIOLS56 and an RBD-5 mAb (IMCAS-L4.65) into a highly potent bispecific antibody, which improved the neutralization efficacy by 100-fold compared to IMCAS-L4.65 and cocktails against Omicron BQ.1 and BQ.1.1 variants. This finding demonstrates that BIOLS56 effectively overcomes the immune evasion of antibodies, enabling robust neutralization against the challenging of Omicron variants.
In summary, this study has introduced a new category of RBD-specific mAbs, named as RBD-8, and has demonstrated that these mAbs recognize a conserved cryptic epitope among sarbecorviruses. Significantly, the study has shown that RBD-8 mAbs exhibit broad neutralization efficacy against all sarbecoviruses and SARS-CoV-2 variants. The mAbs and bispecific antibody identified in this research demonstrate promising potential for clinical treatment applications.
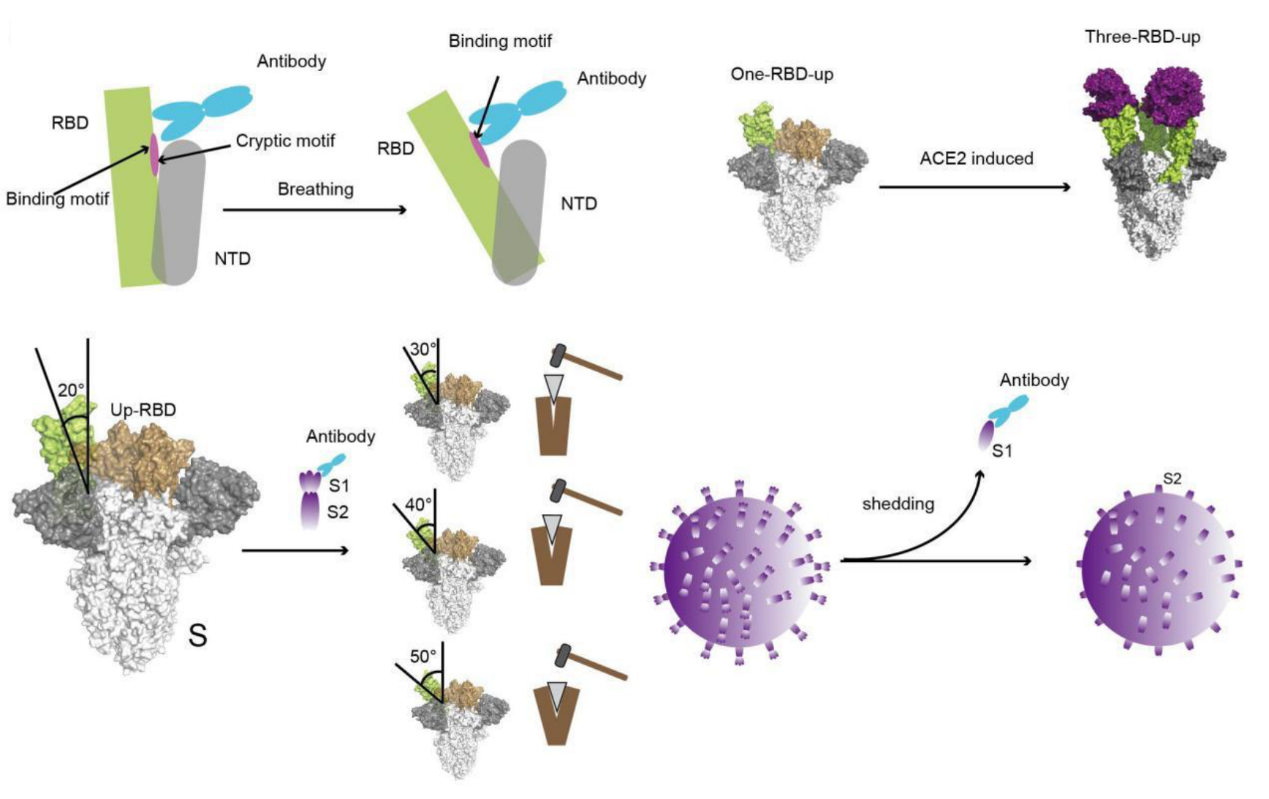
The neutralization mechanism of RBD-8 class antibodies (Image by TIBCAS)
Contact:Prof. Gao Feng
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences.
Phone: 86-22-84861971
Email: gaofeng@tib.cas.cn