Hydrogen transfer reactions play an essential role in both chemistry and biology. A number of hydrogen transfer reactions catalyzed by enzymes have been studied to reveal the importance of hydride transfer (HT) coupled with proton transfer (PT) process(es). Mutations at the residues that participate in the HT or PT routes usually lead to activity decrease or inactivation.
The SUN Zhoutong group from Tianjin Institute of Industrial Biology, Chinese Academy of Sciences explored the crosslink between HT and enzyme activity by using an alcohol dehydrogenase from Thermoanaerobacter brockii (TbSADH) as a model enzyme in the asymmetric reduction of a bulky prochiral ketone (4-chlorophenyl)(pyridin-2-yl)-methanone (CPMK). The phylogenetic analysis showed that the H42 site in the PT step is less conserved in TbSADH. The rational site-directed mutagenesis resulted in the discovery of mutant T13H42T, which displayed a 9-fold improvement in kcat/Km, with no trade-off in stereoselectivity or thermostability. Furthermore, the reasons for the increased activity of T13H42T were investigated with the aid of molecular dynamics simulations and quantum mechanical calculations. The study indicates that the key residues participating in the catalytic proton transfer process may also serve as hotspots for engineering the activity of ADHs.
This work was supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, the Natural Science Foundation Applying system of Tianjin, and Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project. The research paper entitled “Engineering the hydrogen transfer pathway of an alcohol dehydrogenase to increase activity by rational enzyme design” has been published in Molecular Catalysis. The co-first authors are Ms. JIANG Yingying and Dr. LI Xu, while the corresponding authors are Profs. SUN Zhoutong and QU Ge .
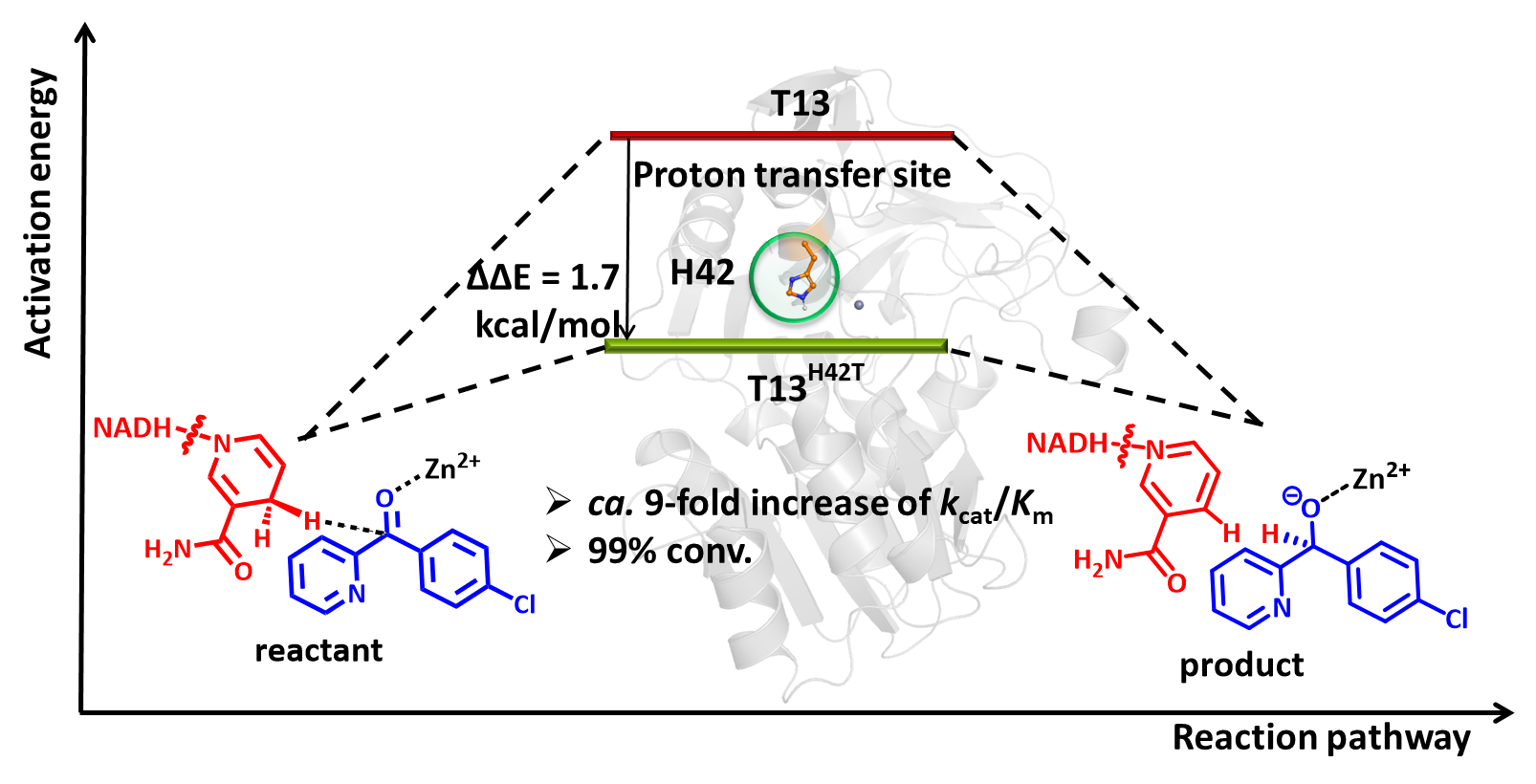
Engineering the hydrogen transfer pathway of TbSADH-catalyzed the asymmetric reduction of CPMK to increase activity.( Image by SUN Zhoutong's group)
Contact:Prof. SUN Zhoutong
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences.
Phone: (0)22-84861981
Email: sunzht@tib.cas.cn