As an important structural element, chiral amino alcohols have been widely used in the synthesis of drugs and biologically active molecules. Asymmetric reductive amination of ketones with free ammonia to produce chiral amino alcohols is a very attractive catalytic reaction. In traditional chemical synthesis, such reactions mainly rely on chemical reducing agents or organometallic catalysts, which are limited by poor stereoselectivity, by-product alcohols, and extreme reaction conditions such as high temperature and high pressure. In previous work, they unearthed and evolved a set of engineered amine dehydrogenases (AmDHs) derived from amino acid dehydrogenases (AADHs), achieved the synthesis of chiral aminoalcohols by asymmetric reductive amination of α-hydroxyketones under mild conditions with water as the only by-product. Despite the high stereoselectivity (>99% ee) of this group of AmDHs in asymmetric reduction, their activities have yet been fully exploited.
For this purpose,the SUN Zhoutong group from Tianjin Institute of Industrial Biology, Chinese Academy of Sciences, improved the activity of the engineered amine dehydrogenase (SpAmDH), a double–site (K68S/N261L) combinatorial leucine dehydrogenase derived from Sporosarcina psychrophila. The combinatorial active-site saturation test/iterative saturation mutagenesis (CAST/ISM) strategy was adopted for evolution. After three rounds of mutation and screening, the mutant wh84 with the best catalytic performance for the substrate 1-hydroxy-2-butanone was obtained, its TTN (32108) and kcat/Km (0.346 s-1mM-1) were increased by 3.2 and 3.9 times compared with the starting enzyme, and still maintained high stereoselectivity (>99% ee). It can also catalyze 100 mM 1-hydroxy-2-butanone to obtain (S)-2-Aminobutanol with an efficiently conversion of 99%. This work expands the biocatalytic toolbox of asymmetric reductive amination and provides technical support for the activity modification of other AmDHs.
The above research has been funded by the National Natural Science Foundation of China, and the relevant results entitled " Biosynthesis of Chiral Amino Alcohols via an Engineered Amine Dehydrogenase in E. coli " has been published online in the international journal Fronters in Bioengineering and Biotechnology. TONG Feifei, a visiting master student from Anhui University, QIN Zongmin, a PhD student at Tianjin Institute of Industrial Biology, and WANG Hongyue, a former master student at Tianjin Institute of Industrial Biology, are the co-first authors of the paper. Prof. SUN Zhoutong and Prof. XIAO Yazhong (from Anhui University) are the co-corresponding authors.
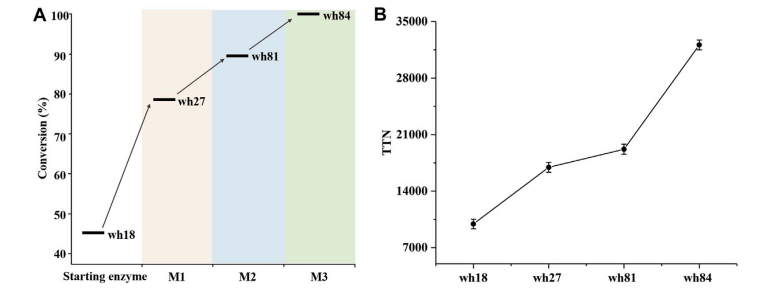
(A) Conversion and (B) TTN analysis of the key variants for catalytic substrate 1-hydroxy-2-butanone ( Image by TIBCAS )
Contact:
Prof. Dr. SUN Zhoutong
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences.
Phone: 0086-22–84861981
Email: sunzht@tib.cas.cn