Chiral amino alcohols are essential building blocks for the pharmaceutical industry (such as the anti-tubercular drug Ethambutol, the anti-AIDS drug Dolutegravir, and the antibacterial drug Pazunoxacin). To date, they are mainly synthesized by conventional chemical methods or enzymatic resolution, whilst the former depend on expensive transition metal catalysts and conversion of the latter is limited to 50%. Alternatively, Amine dehydrogenases (AmDHs) can asymmetrically reduce prochiral hydroxyketones with low-cost ammonia to chiral amine alcohols and water as byproduct, using NAD(P)H as cofactor under mild conditions with 100% theoretical conversion, which is an ideal green synthesis route. However, there were only a handful of relevant reports. How to quickly mine and obtain high-performance AmDHs is challenge in the field of green biosynthesis of chiral amino alcohols.
Prof. SUN Zhoutong’ group from Tianjin Institute of Industrial Biology, Chinese Academy of Sciences, has obtained a series of novel AmDHs through gene mining technology. These AmDHs can efficiently catalyze 1-hydroxy-2-butanone to (S)-2-amino-1-butanol with 19~99% conversions and ee > 99%. Interestingly, the novel AmDHs can also realize the asymmetric reductive amination of a β-hydroxyketone to the β-hydroxy amine (ee >99%). This research provides efficient biocatalysts in the synthesis of chiral amine alcohols. The study entitled “Data mining of amine dehydrogenases for the synthesis of enantiopure amino alcohols” has been published online in Catalysis Science & Technology.
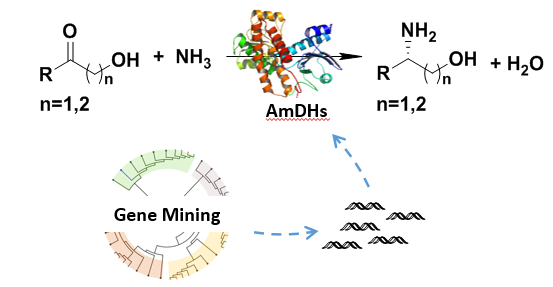
Mining of new amine dehydrogenases for the synthesis of chiral amine alcohols(Image by Prof. SUN Zhoutong’ group)
Contact:Prof. Dr. SUN Zhoutong
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences.
Phone: (0)22–84861981
Email: sunzht@tib.cas.cn