Yellow fever (YF) is an acute infectious disease caused by yellow fever virus (YFV), and it is transmitted mainly by mosquito bites. Infection with YFV can lead to high mortality and morbidity. This disease can be prevented by inoculating YF vaccine (YF-17D attenuated vaccine). However, factors such as the vaccine shortage and coverage of vaccination have led to the recent outbreaks in tropical regions of Africa, Central America and South America. In 2016, imported YF cases were reported in China. There is no specific treatment for YF, mainly for symptomatic treatment and supportive treatment. In recent years, monoclonal antibodies as a drug candidate for the treatment of viral infectious diseases have shown great advantages and potential applications.
The envelope (E) protein of YFV mediates virus entry into host cells and is the main target for neutralizing antibody. Neutralizing antibodies against YFV can inhibit virus infection by inhibiting virus adhesion to host cells or fusion of viral envelope and cell membrane.
Recently, the research team of protein engineering and vaccine of Tianjin Institute of Industrial Biology, CAS, led by Chinese Academician George F. Gao has made important progress in the structure research of YFV E protein and the mechanism of a protective human monoclonal antibody, 5A.
Prof. Gao and his team first solved the crystal structures of soluble E protein (sE) of YF-17D in both pre- and post-fusion forms. They discovered that the overall structure of sE in the pre-fusion state resembles the structure of other flavivirus E proteins. At an acidic pH, YFV sE binds to liposomes and is rearranged as trimers. The trimer formed by YFV sE retains most of its folded structures in all three domains, but there are major rearrangements in the relative orientations of the domains through flexion of the inter-D linkers.
Moreover, they conducted the study of the function, structure and neutralization mechanism of YFV-specific neutralizing mAb, 5A. They found that 5A displayed extremely high neutralization activity against YFV and can protect the mice against lethal challenge of YFV. Furthermore, they solved the crystal structures of 5A complexed with sE of YFV in both pre- and post-fusion forms. The complexes structures reveal that 5A, acting as a “double lock”, binds both pre- and post-fusion sE proteins. Further functional experiments have also demonstrated that 5A can play a role in several steps of virus infection: blocking the virus adsorption on host cells, inhibiting the conformational change of E protein during membrane fusion. These findings will be instrumental for immunogen or inhibitor design.
This study titled "Double lock of a human neutralizing and protective monoclonal antibody targeting the yellow fever virus envelope" has been published online in Cell reports. This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) (grant XDB29010202), the National Key R&D Program of China (grant 2018YFC1200603), the National Natural Science Foundation of China (NSFC) (grants 31600138 and 31771009), and the Natural Science Foundation of Tianjin (grants 15JCQNJC44400 and 17YFZCSY01090). This work was also supported by the National Science and Technology Major Project (grants 2018ZX10101004002004 and 2016YFE0205800).
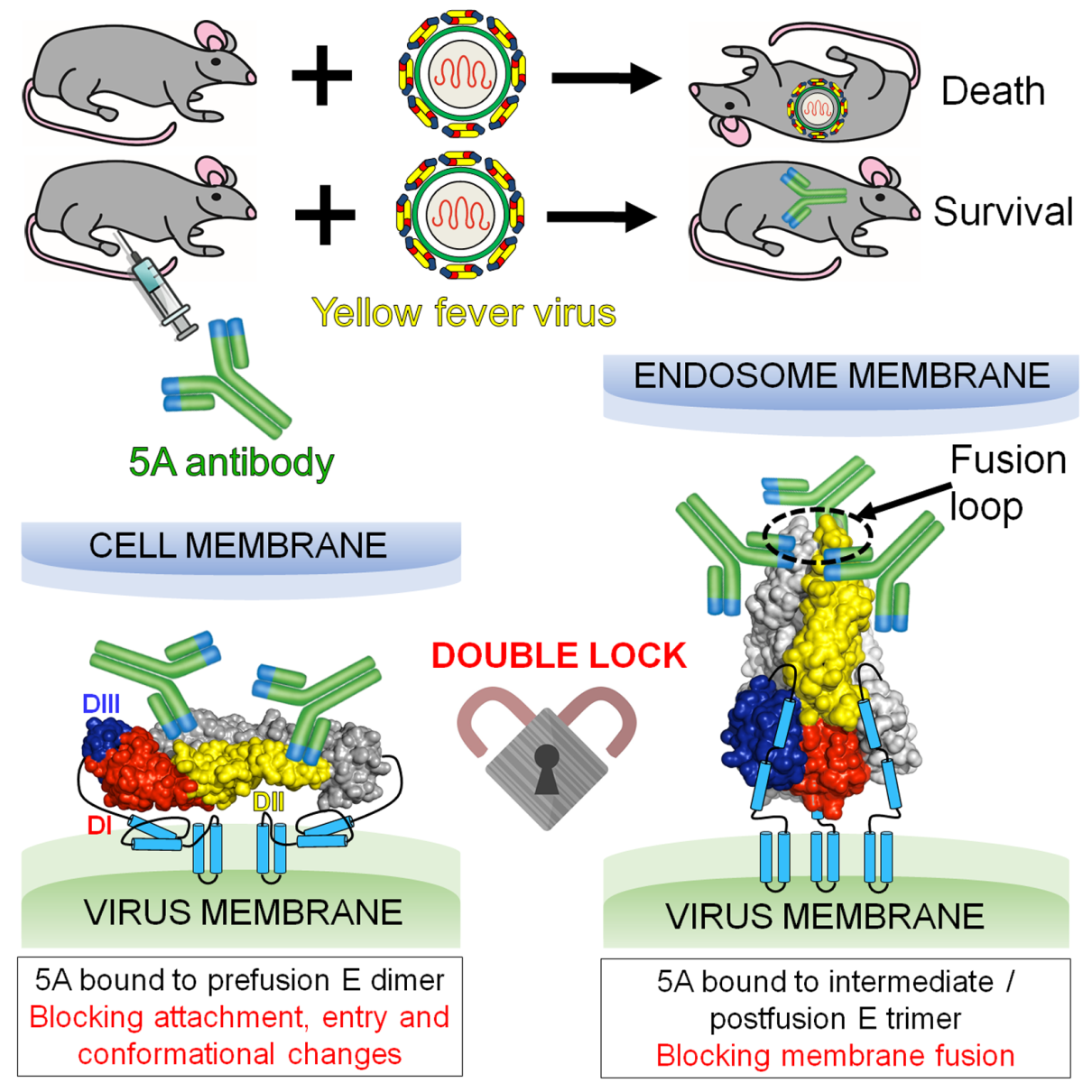
A human mAb, 5A acts as a "double lock" to neutralize YFV
Contact:
Dr. XIAO Haixia
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences.
Email: xiao_hx@tib.cas.cn