Recently, deep investigation was made by Chinese scientists into the functional roles of residues surrounding the csnTS catalytic and substrate-binding sites of chitosanase from Bacillus sp. TS, which belongs to the GH-8 family. Being a kind of oligosaccharide with potential biological activities in several fields, chitosan oligosaccharides can inhibit growth of fungi and bacteria, produce anti-tumor biomedicine and immunity-enhancing factors in the pharmaceutical industry, induce production of phytoalexin in higher plants, and can be used as food additives. Chitosanases (EC 3.2.1.132), which were discovered in bacteria, fungi, viruses and plants, are capable of catalyzing hydrolysis of the glycosidic bonds in chitosan and can be used to produce chitosan oligosaccharides. They represent a diverse group of enzymes classified as members of several different families on the basis of amino-acid sequence homology. Over the past decade, chitosanases from the glycoside hydrolase family 46 (GH46) have been well characterized; however, chitosanases from other families remain poorly understood to date. The Laboratory of Structural Bioinformatics and Integrative System Biology led by Professor SONG Jiangning at Tianjin Institute of Industrial Biotechnology (TIB), Chinese Academy of Sciences (CAS) has been doing research into residues surrounding the csnTS catalytic and substrate-binding sites . Residue Asp318 is in the vicinity of the active site and substrate-binding domain and is highly conserved throughout the GH8 family, especially in chitosanases. Site-directed mutagenesis indicated that although D318N mutant did not affect enzyme activity, other Asp318 mutations resulted in significant loss of catalytic activity. Detailed investigations suggested that Asp318 might play a dual role in regulating csnTS functions specifically involved in both catalytic activity and substrate-binding capability. A research article entitled “A highly conserved aspartic acid residue of the chitosanase from Bacillus sp. TS is involved in the substrate binding” has been recently published in the Applied Biochemistry and Biotechnology Journal. Dr. Zhou Zhanping is the first author of this paper. This work was financially supported by grants from the Knowledge Innovation Program of CAS (KSCX2-EW-G-8), and Tianjin Municipal Science & Technology Commission (10ZCKFSY05600). 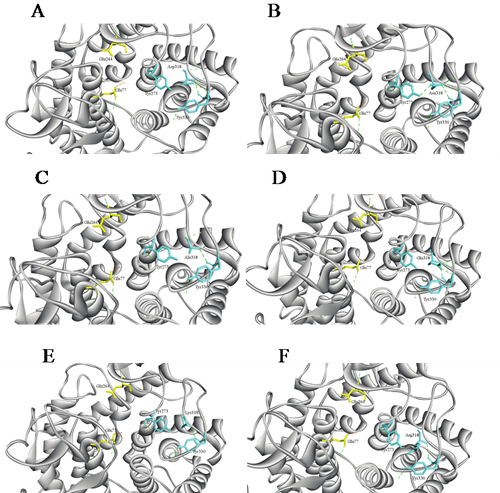
Changes in intermolecular interactions of CsnTS following the single-site mutation of Asp318( Image by SONG Jiangning's Group) Contact: Prof. SONG Jiangning Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences Song_jn@tib.cas.cn |