Plant receptor-like kinases (RLKs) constitute a large family of receptors coordinating develop-mental programs with adaptation to environmental stresses including immune defenses BRI1-ASSOCIATED KINASE 1 (BAK1), a member of the plant RLK family, forms receptor complexes with multiple RLK proteins including BRI1, FLS2, EFR and BIK1 to regulate responses to growth hormones or PAMPs. RLK activation and signal initiation involve protein complex formation and phosphorylation/dephosphorylation between BAK1 and its interacting partners.
To gain new insight into how phosphorylation contributes to BAK1-mediated signaling specificity, the researchers, who from the Laboratory led by Professor SHUI Wenqing at Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, first mapped the phosphorylation patterns of BAK1 associated with different RLK partners (BRI1, FLS2, EFR and BIK1).
Quantitative phospho-pattern profiling by label-free mass spectrometry revealed that differential phosphorylation patterns of RLK partners resulted from altered BAK1 phosphorylation status. More interestingly, the study of two BAK1 mutants (T450A and C408Y) both showing severe defect in immune defense yet normal growth phenotype suggested that varied phosphorylation patterns of RLK partners by BAK1 could be the molecular basis for selective regulation of multiple BAK1-dependent pathways.
Taken together, this phospho-pattern profiling strategy allowed for explicit assessment of BAK1 kinase activity in different RLK complexes, which would facilitate elucidation of BAK1 diverse functions in plant development, defense, and adaptation.
The study entitled “Assessment of BAK1 activity in different plant receptor-like kinase complexes by quantitative profiling of phosphorylation patterns” has been published in Journal of Proteomics (2014 Jun 20). WANG Yilin (a graduate student trained by TIB and Nankai University) is the first author of this paper. This work was supported by the National Natural Science Foundation of China (31170782) and Tianjin Natural Science Foundation in China (11JCYBJC25500).
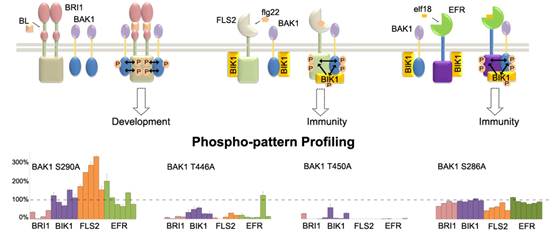
Phosphorylation-pattern profiling approach for LC-MS quantitative assessment of BAK1 kinase activity(Image by SHUI Wenqing's group)