Tuberculosis (TB) is an infectious disease caused by Mtb. At present, there are about 10 million new TB cases globally every year, and about 1.2 million people died of TB. Of note, The emergence and spread of multi-drug and extensive drug resistance Mtb strains challenges the control and treatment of TB.
As we know, Mtb develops drug resistance through various mechanism, with efflux pump-mediated drug extrusion being one of the majors. EfpA was the first MFS (facilitator superfamily) efflux pump identified in Mtb, and it is essential to the bacterium’s survival. Previous studies have reported that the expression ofEfpA contributes to resistance against both first- and second-line anti-TB drugs, including isoniazid, rifampicin, ethionamide, amikacin and fluoroquinolones. More importantly, inhibitors targeting EfpA have been shown to inhibit the growth and kill Mtb, highlighting EfpA as a promising drug target However, the exact function of EfpA and the mechanism of EfpA-dependent drug resistance remain largely unknown.
In this recent study published in PNAS, the research group of Protein Engineering and Vaccines from Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, along with its collaborators published a research article titled “Structure and function of Mycobacterium tuberculosis EfpA as a lipid transporter and its inhibition by BRD-8000.3”. These researchers solved the cryo-EM structure of EfpA in complex with multiple lipids at a resolution of 2.9 Å revealing that EfpA assembles into an antiparallel dimer, with each protomer consisting of 14 transmembrane helices. Additionally, a range of lipids bound to EfpA were detected by LC-MS/MS, among which phosphatidic acid (PA) matches best with the densities in the central cavity and the lateral fenestration. Notably, residues S191 and T324 of EfpA, interact with the head group atoms of PA through polar interactions. Furthermore, using a lipid flipping assay, this study demonstrates that EfpA functions as a lipid transporter, and its transport activity of EfpA can be inhibited by BRD-8000.3.
Moreover, this study solved the cryo-EM structure of EfpA in complex with BRD-8000.3, revealing that two BRD-8000.3 molecules occupy the lipid binding site at the dimer interface. Based on the structure analysis, mutations at the position, V319 of EfpA were constructed, and it turned out that the expression level of V319F mutant rather than V319M increased more than tenfold compared to wild type. Moreover, the oligomeric state of V319F mutant is also altered, which may contribute to its resistance to BRD-8000.3.
In summary, this study reveals the actual function of EfpA as a lipid transporter and uncovers the structural basis for its interaction either with both PA and the drug molecule, a BRD-8000.3. Clearly, these findings suggest a potential role for EfpA in the synthesis of Mtb cell envelope, and the lipid transport activity may be associated to the drug resistance caused by EfpA. The structural and functional characterization of EfpA will facilitate the development of anti-TB drugs against this promising therapeutic target.
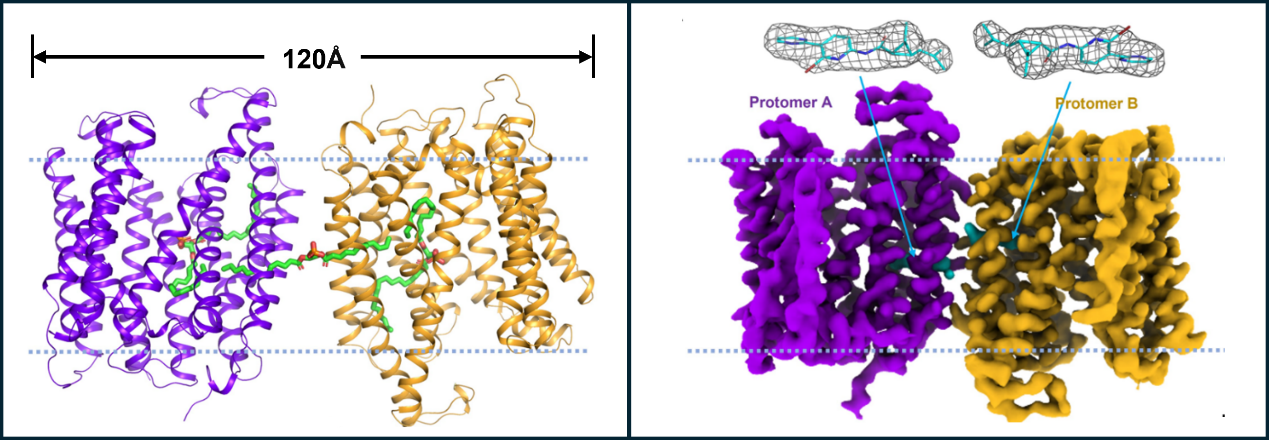
Figure 1. The structures of EfpA in complex with lipids (left) and with the inhibitor (right). ( Image by TIBCAS)
Contact:Prof. GAO Feng, XIAO Haixia & GAO Fu
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences.
Phone: 86-22-84861971
Email: gaofeng@tib.cas.cn, xiao_hx@tib.cas.cn, gaof@im.ac.cn