The recovery of heavy metals by applying microorganisms is an established biotechnological technique. The mobilization of metal cations from insoluble ores by biological oxidation and complexation processes is referred to as bioleaching. For the lean grade ores and tailings, which are supposed to be the future resources, bioleaching are still in their infancy or confined in the laboratory scale. The potential of heterotrophiles for leaching/beneficiation are yet to be fully exploited along with its major challenges in process design remain unresolved. The idea nurtured, expertise developed in the laboratories should find their way to get transformed in to feasible technology, then only the commercialization of such metal recovery processes from lean grade ores or overburdens will be shimmering as a reality. To meet the present-day industrial need for manganese, it is necessary to develop manganese ore deposits containing 9–25% manganese. This content is too low for currently existing processing methods, and the production is unprofitable. Therefore, new methods are sought for manganese recovery from low-grade ores and for concentration of ores. The chemoautotrophic bacteria Acidithiobacillus thiooxidans and Sulfobacillus sp. herein simultaneous leach pyrite and pyrolustie synergistically for manganese recovery. Microbial flora, pyrite, pyrolusite and sulfuric acid were put into one system to run reaction. Maximal recovery ratio was 92.43% after 12 days leaching. To meet industrial demand, current bioleaching system must be optimized. Fractional leaching method was introduced and new reactor optimized for energy saving was constructed (Figure 1). The technique contained two steps. In first step, bacteria leached pyrite in biological favoring condition and fermentation broth was formed. The broth was used to leaching pyrolusite in next step. Mature lixivium and high concentration of active bacteria in fermentation broth guaranteed fast reaction in second step (Table 1). Figure 1 Redesigned reactor for bioleaching 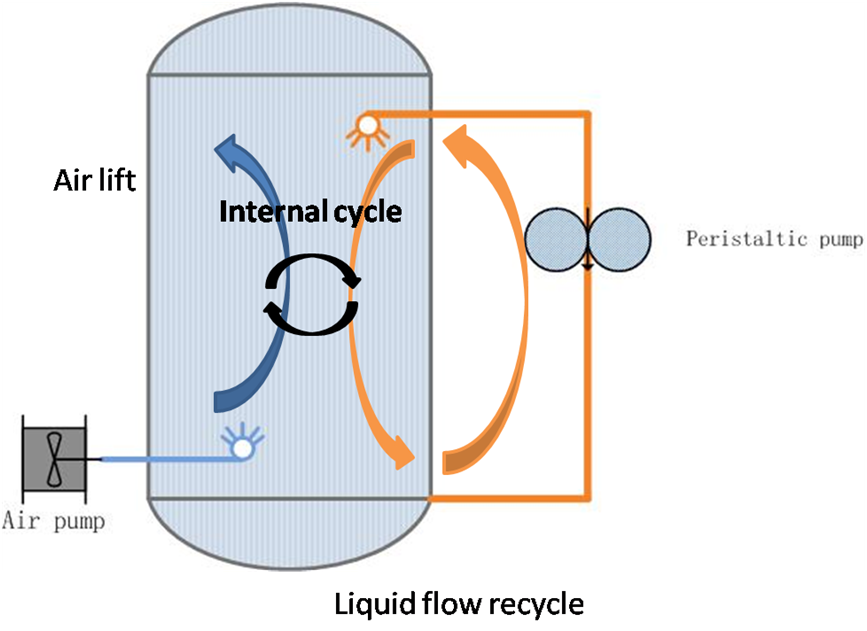
Table 1 Parameter of Fractional bioleaching of Mn
|
Parameter |
Fermentation step |
Mn recovery step |
|
Temperature |
30 |
30~60 |
|
Liquid vs solid |
100 : 1 |
3 : 1 |
|
Reaction period |
4~5 days |
With 24h |
|
Mn recovery ratio |
- |
Up to 96% | Relative to direct bioleaching, shorter reaction period and lower liquid vs solid in Mn recovery step make existing production recycle process being applied (Table 2). Table 2 Comparison of Fractional leaching and direct bioleaching
|
Parameter |
Fractional leaching |
Direct bioleaching |
|
Pyrite |
0.17 |
0.3 |
|
Sulfuric acid |
0.13 |
0.18 |
|
L vs S |
3:1 |
100 : 2 |
|
Leach period |
24h |
12 days |
|
Mn recovery |
Up to 96% |
Up to 92% | |